
The estimated prevalence of TSC in recent studies falls in the range of 1/6000 to 1/10,000. In addition, TSC can also cause cognitive nerve deficits and behavioral and developmental disorders, such as epilepsy.
Mtmr inhibitor skin#
Tuberous sclerosis complex (TSC) is an orphan disease that affects many organ systems to varying degrees and is typically characterized by benign tumors of the skin (facial angiofibroma), brain (subependymal giant cell astrocytoma), kidneys (angiomyolipoma), heart (rhabdomyoma), lungs (lymphangioleiomyomatosis) and retina (optic nerve tumor). Unlike the risks of upper respiratory tract infections and nasopharyngitis, mTOR inhibitors seem to increase the risk of stomatitis, mostly grade 1 and 2. In view of the efficacy and safety associated with tumor and seizure frequency in the TSC patients, mTOR inhibitors is a good therapeutic choice. In contrast, patients who did and did not receive mTOR inhibitors experienced similar adverse events, such as upper respiratory tract infections (RR = 1.08, 95% CI = 0.81,1.45, P = 0.59) and nasopharyngitis (RR = 0.86, 95% CI = 0.60,1.21, P = 0.38). Regarding safety, compared with patients who did not receive mTOR inhibitors, those who did had a higher risk of suffering stomatitis (RR = 3.20, 95% CI = 1.49,6.86, P = 0.003). Compared with the placebo, mTOR inhibitors significantly reduced seizure frequency (RR = 2.12, 95% CI = 1.41,3.19, P = 0.0003). The included RCTs were analyzed using RevMan 5.3, which was provided by the Cochrane Collaboration.
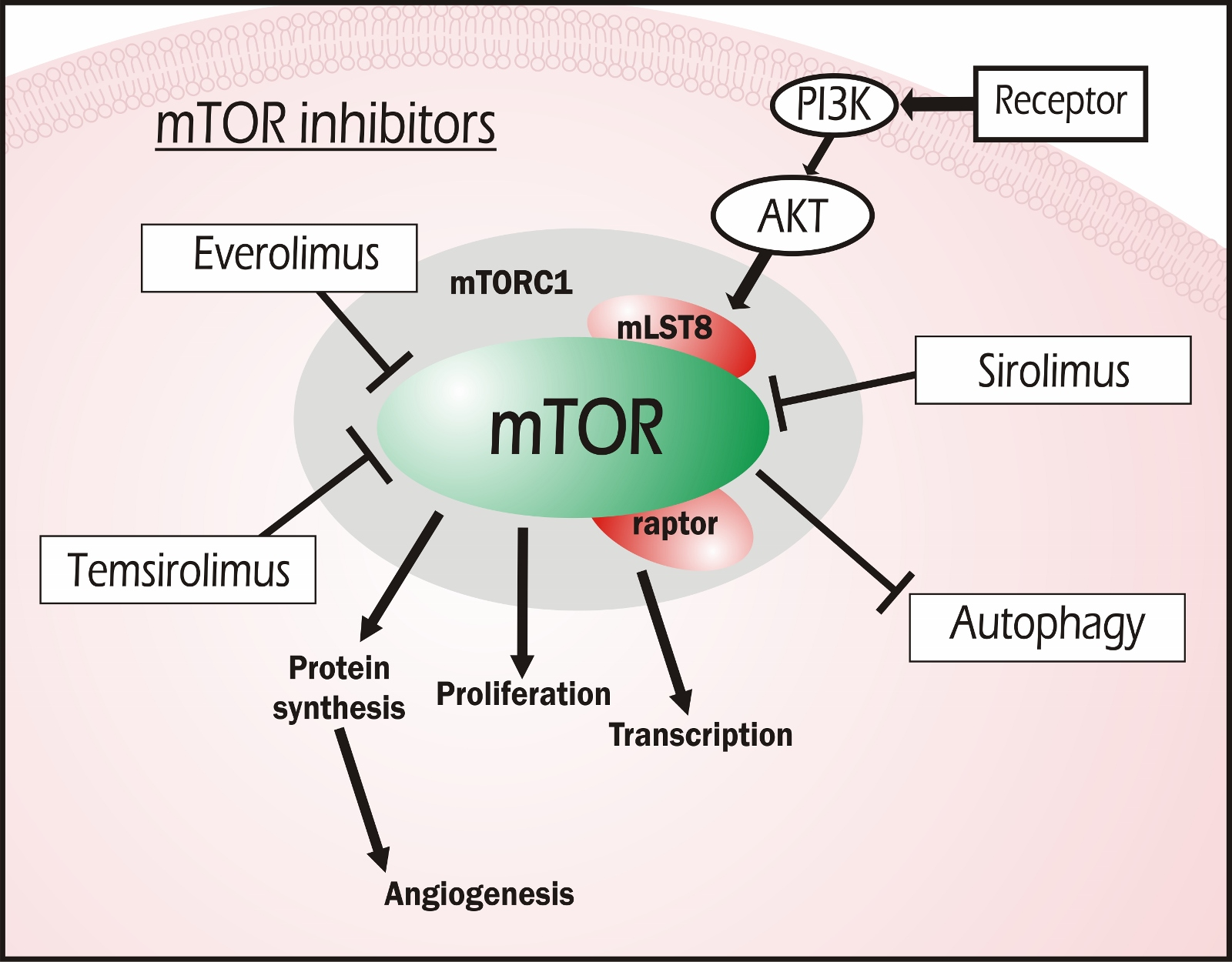
Two researchers screened articles, assessed the risk of bias and extracted data independently. The endpoints of the study were the tumor response rate and seizure frequency response rate (the proportion of patients achieving a ≥ 50% reduction relative to the baseline). We performed a systematic search of major electronic databases (PubMed, EMBASE, Cochrane Library and WanFang, CNKI, and VIP databases) to identify randomized controlled trials (RCTs) and quasi-randomized studies from the date of database inception to November 2017 the Chinese Food and Drug Administration and were also searched for unpublished studies. The aim of the present study was to evaluate the efficacy and safety of mTOR inhibitors for improving the clinical symptoms of TSC. The treatment of tuberous sclerosis complex (TSC) using mammalian target of rapamycin (mTOR) inhibitors is clinically promising.


 0 kommentar(er)
0 kommentar(er)
